|
Blue, Green, and Opaque Red
The addition of specific coloring agents allows
the hue of glass to be altered a great deal. The intensity of natural
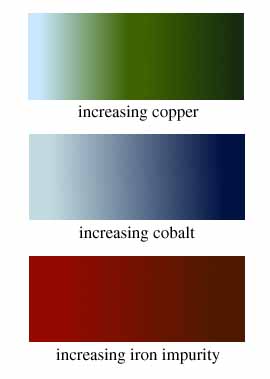
aquablue and green in a vessel is increased by the addition of copper,
in some form, and an oxidizing atmosphere in the furnace. An aquablue
gives way to a dark green as the amount of copper (as azurite, chalcopyrite,
or scrap bronze) increases from 2% to 13%.
A dark royal blue is obtained using a copper
mineral contaminated by cobalt. The presence of as little as 0.1%
cobalt can cause intense coloration of glass. Other impurities that
occur in cobalt ores, e.g. manganese, iron, and nickel, will darken
the blue even further.
Opaque red glass is formed in the same way, but
by the use of a reducing atmosphere in the furnace. The copper is
precipitated out as minute, needle-like crystals of cuprous oxide.
If the copper content is above 10%, these crystals scatter through
a colorless matrix and give the appearance of a bright, blood-red
glass. If some of the copper additive is still dissolved in the
glass, however, a muddy brown coloration results.
|
|
 |

