I. Restoration of the Silver
THE metallurgists of early times, as is well known, were able to produce bronzes of a composition and quality that have never been surpassed. In fact, their alloy containing approximately 90 per cent copper and 10 per cent tin is regarded as the best bronze composition for many purposes today. Their gold and silver, on the other hand, are not of the degree of purity that modern methods of refining permit, yet their artistic value does not suffer because of this. The delicate design of many gold and silver objects and the apparent skill of the workman are frequently such as to command one’s admiration. This is particularly true of the silver objects from Ur, but one cannot fully appreciate these gems without first revealing the details by appropriate means of restoration.
The silver from Ur was encrusted with a thick layer of silver chloride, some so-called secondary silver from which the copper had been leached out, and in some cases of copper, so that it was not only difficult to appreciate their value but often quite impossible to distinguish their shape or details. In attempting to restore them, the very excellent methods of Dr. Alexander Scott, F.R.S., of the British Museum, who employed formic acid with marked success, and of Dr. Colin G. Fink of the Metropolitan Museum, who developed the electrolytic caustic soda treatment, were used whenever possible. However, no general method of treatment will apply in all cases and a number of modifications were necessary. This can best be illustrated by a brief description of the treatment given a few of the objects.
Silver Libation Ewer
This silver ewer (Plates XIII and XIV), which stands eight to ten inches high, had been filled with plaster to prevent it from being further crushed. The spout had been broken. The entire object was covered with a hard brown crust, essentially of silver chloride. After giving the electrolytic and then the formic acid treatment, most of the crust could be removed mechanically. Badly stained areas were rubbed with a paste of zinc dust and weak sulphuric acid. The object was then gently brushed, washed, dried and lacquered. The metal was too thin and brittle to permit annealing and reforming.
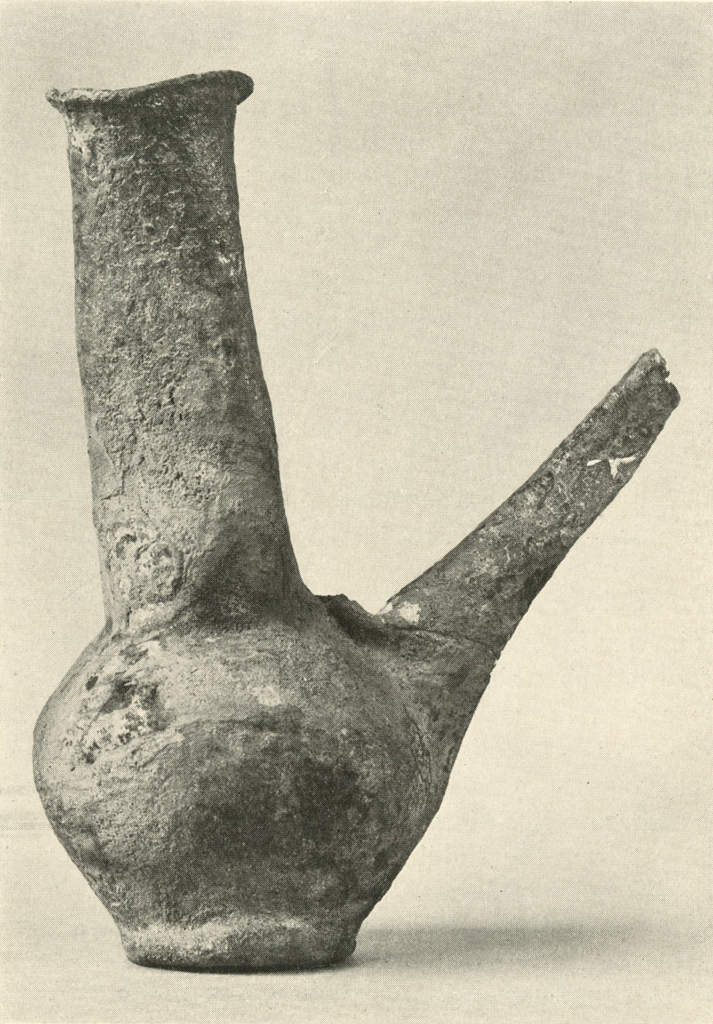
Museum Object Number: B17083
Image Number: 8192
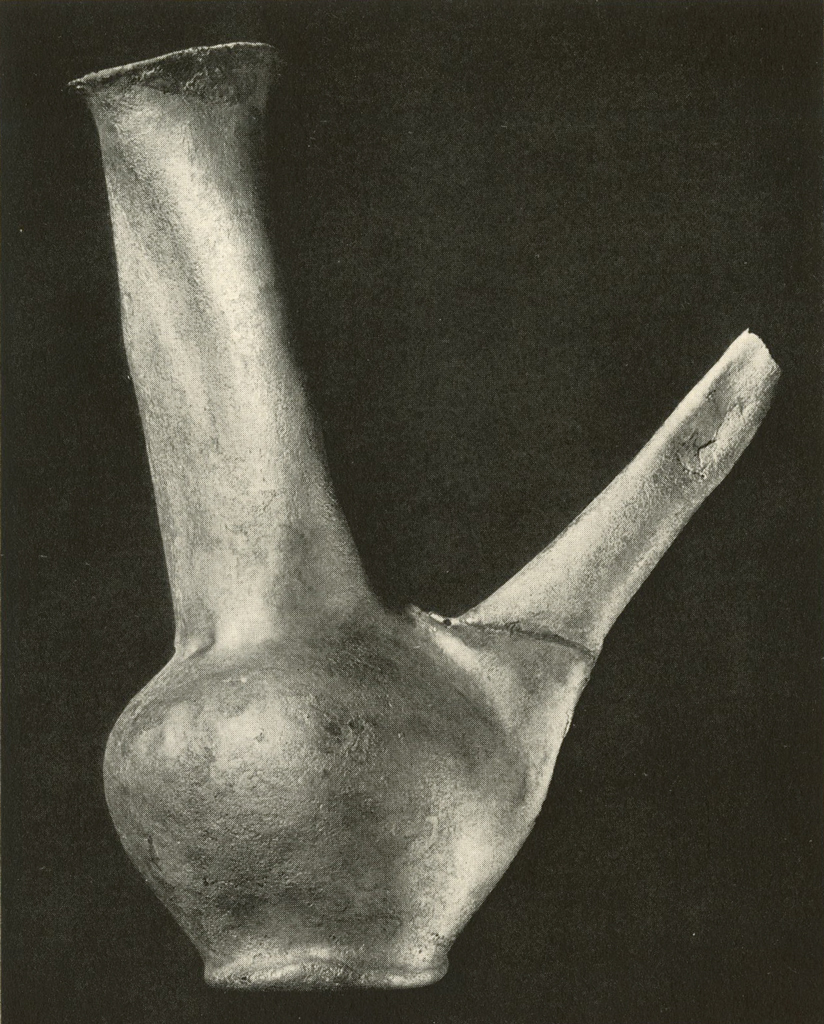
Museum Object Number: B17083
Image Number: 8195
Silver Bowl

Museum Object Number: B17067
Image Numbers: 8191, 8218
The silver bowl (Plate XV) had been nested in another bowl, so that a portion of the outside was almost perfectly preserved while the rest of the surface, both inside and outside, was severely corroded. The uncorroded area was smooth and showed no hammer marks, so that the article had no doubt been burnished after hammering into shape, as determined by evidence presented at the end of this paper.
The restoration treatment in this case was essentially the same as that given the previous object. It is interesting to note the distinctive mark on the outside of the bowl, which was not visible until the corrosion products had been removed. This stamp appears on many of the gold and silver objects and may have been used to identify the work of a particular silversmith in a similar manner to the “Hall Marks” which are such an interesting feature of the silver of a few hundred years ago.
Silver Antelope’s Head and Bracelet
This interesting conglomerate (Plate XVI) was studied for some time before it was realized that the object holding the cockleshell might be a head of some horned animal. It was attached very securely to what was believed to be a solid silver ring. The object was given a long electrolytic treatment, followed by repeated boiling in formic acid, and brushing. A sharp pick was used to remove the loosened crust from the fine lines of the head. After gentle brushing it was washed, dried and lacquered.
The ring, after it had been separated from the head, while boiling in formic acid, was treated in a similar manner to the head and proved to be a bracelet of several turns of silver.
Several shells, gold and silver beads, and a lapis eye were also obtained. The large white cockleshell contained blue cosmetic similar to that reported upon elsewhere in this volume.
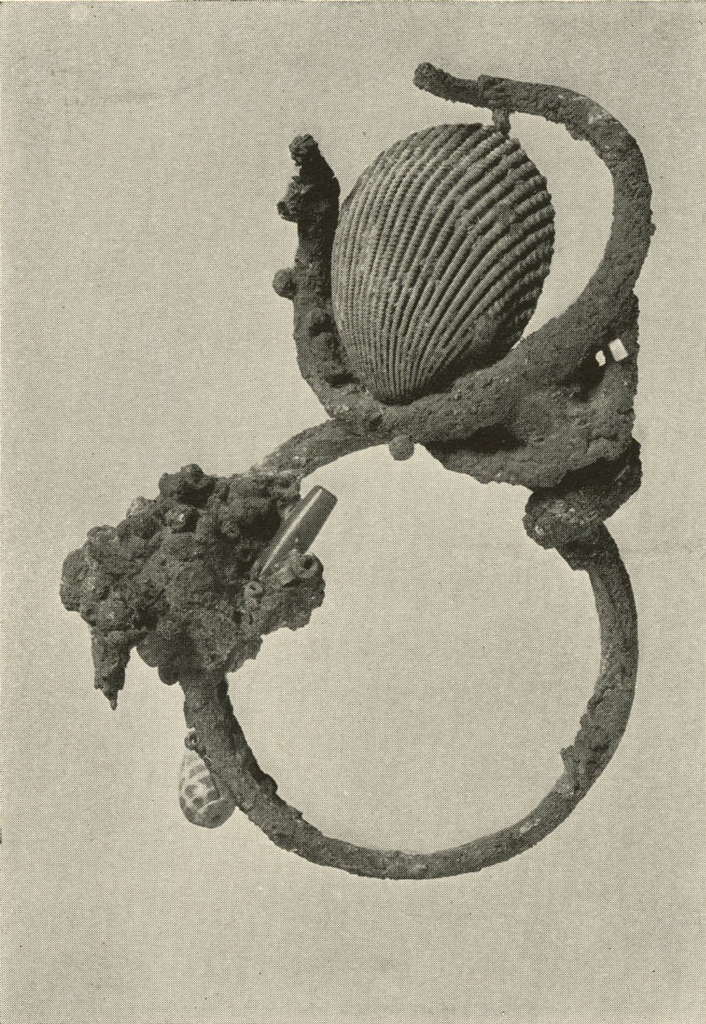
Image Number: 8242

Museum Object Number: B17716
Gold Inlaid Silver Bowl

Museum Object Number: B17077
Image Numbers: 8191, 8239
This bowl (Pl. XVII) had evidently been given some treatment prior to the time that it was received. The silver was very rough as the result of corrosion and the gold or “speculum” inlay was slightly tarnished and loosely adherent in places. The usual chemical procedure could not be followed for fear of losing the inlay. A mild treatment with formic acid and gentle brushing improved its appearance quite noticeably, after which it was washed, dried and lacquered. The twisted silver handle was too corroded to permit restoration.
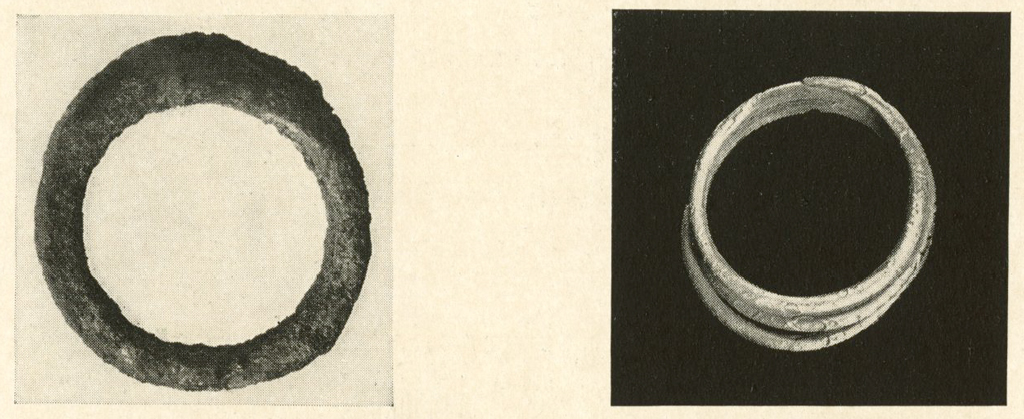
Silver Rings
A number of silver rings were treated (page 255). In some cases there was a very hard crust of secondary silver surrounding a weak porous core of metal. The crust could not be removed by mechanical means without danger of breaking the object and was quite resistant to any of the reagents used to remove the outer layer of chloride.
In other cases the crust was almost entirely silver chloride and after the usual electrolytic and formic acid treatment, boiling in ammoniacal sulphite solution containing small quantities of copper assisted in removing the crust. In severe cases soaking in sodium thiosulphate solution worked well, but the metal was apparently weakened if allowed to remain in the solution too long.
Silver Beads
A large quantity of silver beads were badly corroded and sometimes firmly stuck together (Pl. XVIII). These could not be wired for the electrolytic treatment. They were usually given a soaking in ammonium hydroxide (30% by volume), boiled in formic acid and then given one or both of the other treatments, namely, boiling in sulphite solution or soaking in thiosulphate. When the beads were separated and most of the corrosion product was removed, they were redrilled, strung on wires and brushed. Those that required no further treatment were washed, dried and lacquered.
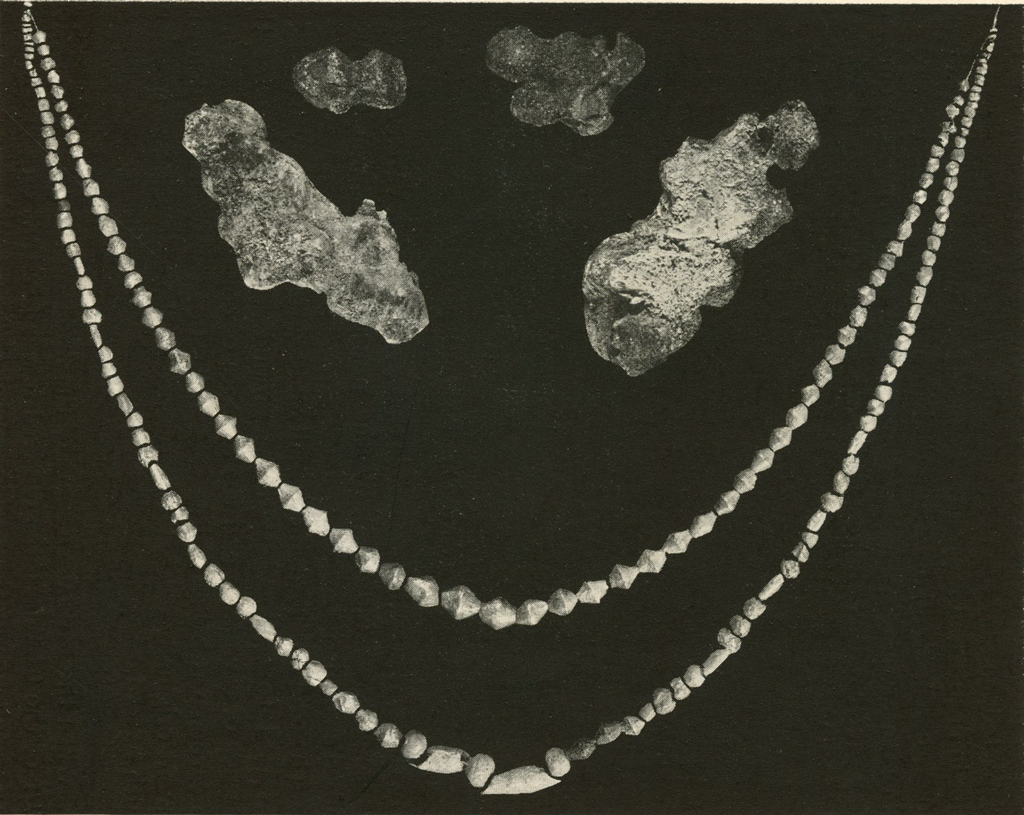
Museum Object Numbers: B17714
Image Numbers: 8236, 8232
II. Metallurgical Notes
Not much is known about the methods of working metals in these early times, but to reproduce the silver bowl shown in Plate XV would require no ordinary amount of skill even with modern methods. The silver alloy would first have to be prepared and cast into convenient form. It would then be alternately heated in a furnace (annealed) and rolled until a flat sheet of the desired thickness is obtained. The silversmith would carefully study the shape of the object to be produced and then proceed to cut a pattern out of the flat sheet metal. He would then prepare forms upon which the metal would be hammered to the finished shape. In accomplishing this, at least three to five annealing operations would be required, depending on the skill of the workman, in order to keep the metal soft enough to work into shape.
If the silver articles from Ur were produced in a similar manner, the metal would exhibit a structure similar to a modern silver article. Examination under the microscope has shown this to be the case (page 257). The structure is that of annealed metal with numerous cases of twinning indicating previous working. One may conclude, therefore, that the silver alloy was first cast and then alternately annealed and hammered until the desired object was obtained, as twinning can occur only when metal is annealed after being worked.
Silversmiths of today are regarded as the highest type of skilled labour and there is a dearth of them, both on this continent and abroad. To produce a libation urn as illustrated in Plate XIV would be a worthy accomplishment even with modern methods, which is tribute enough to the workman who made it.
III. The Cosmetics of Queen Shubad
Those guardians of the public welfare whose duty it is to see that the requirements of the food and drug laws are upheld would have had front page publicity in the time of Queen Shubad because her lip and eyebrow paint contained large quantities of lead, a trace of which is dangerously poisonous. This rather startling discovery was made while analyzing two samples of cosmetics taken from the tomb at Ur. The other results of these analyses are also of sufficient scientific interest to be given in detail.

One sample of what appeared to be a light blue clay was found to contain large quantities of aluminum phosphate, copper, lead and carbonate, with traces of iron, calcium and silica. One would conclude that this was powdered turquoise, a naturally occurring mineral consisting of hydrated aluminum phosphate with the usual copper impurity in sufficient quantity to colour it blue.
A second sample of black powder similar to antimony or “kohl” was found upon analysis to contain a large amount of manganese and lead, with small quantities of copper, aluminum, phosphate, carbonate, silica and iron. The last six substances were evidently present as turquoise, as described above. The black colour could only be attributed to the manganese, the black oxide of which is a naturally occurring mineral, pyrolusite.
The presence of lead and carbonate in both samples is quite unexpected, as they are not associated with either turquoise or pyrolusite in nature and must have been added purposely. The oxides of lead are coloured and when mixed with the above minerals in powdered form give attractive shades of brown, red and purple. Women of today prefer red for the lips and black for the eyebrows and, while it is known that black, blue and red colors were similarly used in those early times, it is entirely possible that even a greater variety of colour was sought, as indicated by the unusual composition of Queen Shubad’s cosmetics.
The oxides of lead, if originally added for colour, may have been converted to carbonates in the ground, thus accounting for the presence of carbonate and the absence of colour due to its presence, the carbonates being white. Regardless of the form in which this element originally existed, its presence in the cosmetic was a serious health hazard.
——
When the Museum’s share of the finds of the Joint Expedition to Ur of the Chaldees was received it was felt that on the one hand the permanence of the various metal objects could be assured and on the other their appearance greatly improved were they subjected to study by those particularly trained in the subject of metallurgy and chemistry. The assistance of Dr. A. Kenneth Graham, of the Towne Scientific School of the University, was elicited and most generously given. His care in carrying out the exacting processes which scientific knowledge indicated would be advisable, and his researches in the methods employed by the early smiths in fashioning the vessels, have been of the utmost value. The delicate little silver head of an antelope, for example, illustrated in Plate XVI shows what important works of art and early culture can be retrieved from apparently worthless masses of corroded metal, and how valuable to the archæologist is the cooperation of the specialist in modern scientific research.
In the present article Dr. Graham describes the technique and the particular treatment given a few of the more important pieces that benefited by his care. Those who visit the Ur Gallery will, we feel, view with added interest the pieces restored in the course of his work. Their beauty is greatly enhanced, their preservation is assured, and for all time it will be possible to realize how great was the mastery of these early metalworkers. It is but one of the many debts archæology owes, and will increasingly owe, to modern scientific research.
EDITOR.

